Abstract
Background: Acute myeloid leukemia with mutations in TP53 is a particularly aggressive form of the disease with low survival rate and an unmet need for new insights into the biology of mutant p53 in leukemic cells. TP53 mutations, but not deletions, are significantly associated with patient survival 1. This suggests that stable mutant p53 protein may have additional gain-of-function (GOF) activities that fuel cell proliferation and promote cell survival. A deeper understanding of p53-independent functions of Mdm2, especially in the context of mutant p53 malignancies, may reveal new therapeutic vulnerabilities.
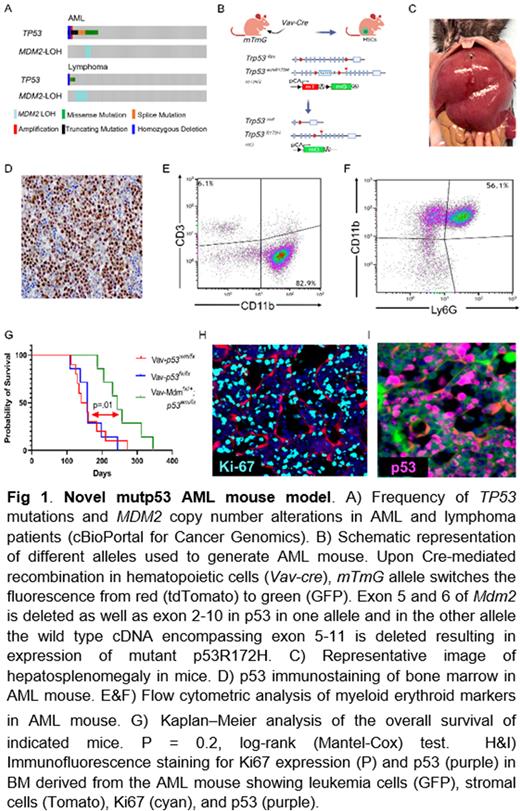
Methods: We determined the frequency of MDM2 LOH and assessed the MDM2SNP309 genotype, in the Pan-Cancer Analysis of Whole Genomes (PCAWG) cohort querying 2,583 patients. To functionally assess the role of MDM2 in p53-mutant hematopoietic stem cells (HSCs), we generated a new p53 mutant AML model by heterozygous deletion of Mdm2 and induction of a Trp53R172H single point mutation in HSCs using Vav-Cre as the Cre driver. We characterized the leukemia cells derived in these mice and further explored the role of Mdm2 in leukemia development.
Results: In AML, MDM2 LOH was concomitant with TP53 missense mutations, but not TP53 deletions or truncations (log2 odds ratio > 3, P < 0.001). This association was not present in lymphomas, in which we found MDM2 LOH and TP53 mutations to be mutually exclusive. Furthermore, MDM2 LOH was significantly associated with lower survival (P = 0.002), suggesting a potential prognostic impact of MDM2 LOH in TP53-mutant AML.
To functionally assess the effect of Mdm2 LOH on AML phenotype, we generated a novel p53-mutant mouse model. Mice harboring the Trp53R172H allele combined with Mdm2 haploinsufficiency developed AML with full penetrance. Immunostaining of BM and spleen cells derived from animals harboring AML displayed strong nuclear expression of mutant p53. Flow cytometric analysis of cells derived from BM showed previously described murine AML phenotype of CD3- , CD11b+, Ly6G+.
To understand the molecular mechanism of myeloid-biased hematopoiesis in Mdm2-haploinsufficient mice, we performed RNA sequencing of BM-derived Lin-/c-Kit+ (LK) HSCs isolated from adult Vav-Cre;Mdm2Wt, Vav-Cre;Mdm2fl/+, and Vav-Cre;Mdm2fl/+;Trp53fl/fl mice. The population of Linneg-c-Kit+ hematopoietic stem/progenitor cells isolated from the bone marrow of Vav-Cre;Mdm2fl/+ mice displayed marked downregulation of cholesterol biosynthesis and mevalonate pathway (-log2 pvalue=20). Homozygous deletion of Trp53 in Vav-Cre;Mdm2fl/+ mice did not rescue the metabolic alterations driven by Mdm2 haploinsufficiency. To examine whether inhibition of cholesterol biosynthesis in HSCs by statins contributes to myeloid differentiation, we isolated LSK cells and treated them with Atorvastatin. Atorvastatin treatment significantly increased myeloid differentiation after 3 days (t test, P = 0.0001), suggesting that the mevalonate pathway plays a role in myeloid differentiation. We also assessed the combinatorial effects of Atorvastatin and nutlin3a in the presence and absence of CoQ. All three cell lines were relatively sensitive to nutlin3a and Atorvastatin, and the combination was highly synergistic in all 3 cell lines. Importantly, CoQ treatment strongly reduced apoptotic cell death, particularly in the Atorvastatin/nutlin3a combination group, suggesting that the inhibition of the mevalonate pathway may enhance MDM2i-mediated oxidative stress by depletion of CoQ. Collectively, these results reveal a role for Mdm2 in myeloid differentiation through the mevalonate pathway and identify combinatorial effects of statins and MDM2i as a rational strategy for treating AML.
Conclusion: We provide clinical evidence that MDM2 loss of heterozygosity is concomitant with TP53 mutations in AML and is associated with shorter survival compared to TP53 mutant patients with diploid MDM2. We present the first p53-mutant AML model, and highlight a new p53-independent function for Mdm2 in vivo in a metabolic switch from cholesterol biosynthesis to oxphos. The p53-mutant AML model may serve as a valuable immunocompetent preclinical model to study the biology and to discover novel vulnerabilities of p53 mutant AML.
Disclosures
Konopleva:Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy; Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Forty-Seven; F. Hoffman LaRoche: Honoraria. Andreeff:Daiichi-Sankyo Inc.: Consultancy, Research Funding; Reata: Current holder of stock options in a privately-held company; AstraZeneca: Research Funding; Oxford Biomedical UK: Research Funding; Oncolyze: Current holder of stock options in a privately-held company; Breast Cancer Research Foundation: Research Funding; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Chimerix: Current holder of stock options in a privately-held company; Medicxi: Consultancy; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; NCI: Membership on an entity's Board of Directors or advisory committees; Pinot Bio: Research Funding; Brooklyn ITX: Research Funding; Glycomimetics: Consultancy; Senti Bio: Consultancy, Research Funding; Syndax: Consultancy, Research Funding; Kintor Pharmaceutical: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal